Arterial blood gasses, or ABGs, are among the more complex assessments performed by clinical healthcare professionals.
Fortunately, there are some easy ways to remember how to decipher these important results.
What is Normal?
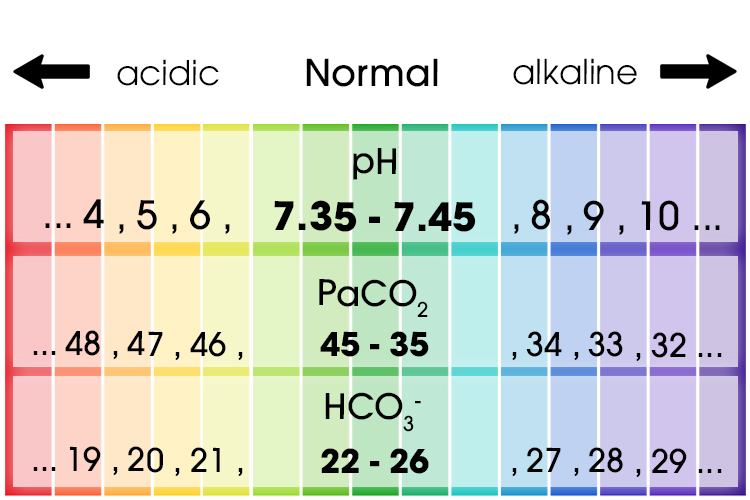
When interpreting ABG results, it is essential to know what ABG values are considered ‘normal’. From this baseline, you can then begin to recognise significant variations in a patient’s results, which could indicate clinical deterioration.
The first value is the pH, which measures how many hydrogen ions (H+) are in the sample. This determines if the blood is acidotic or alkalotic. Normal values for pH range from 7.35 to 7.45.
The next value is the carbon dioxide level. This will tell you if the problem is respiratory in origin, as CO2 is regulated by the lungs. The normal range for PaCO2 is 35 to 45 mmHg.
Finally, bicarbonate ions, or HCO3-, will tell you if the problem is related to metabolic changes in your patient and refers to the renal system. Normal is considered to be from 22 to 26 mmol/L.
Normal ABG Levels
| pH | Hydrogen | 7.35 - 7.45 |
|---|---|---|
| PaCO2 | Carbon dioxide | 35 - 45 mmHg |
| HCO3- | Bicarbonate | 22 - 26 mmol/L |
(Castro et al. 2024)
Put simply, when the numbers in an ABG result fall outside of these ranges, you can then determine what type of problem the patient is experiencing.
pH: Acidic or Alkalotic?
If the ABG results reveal pH numbers are not within the normal range, the patient’s pH level is either acidotic or alkalotic.
The lower the number, the more acidotic the patient is. For instance, a pH of 3 is severely acidotic and requires emergency intervention.
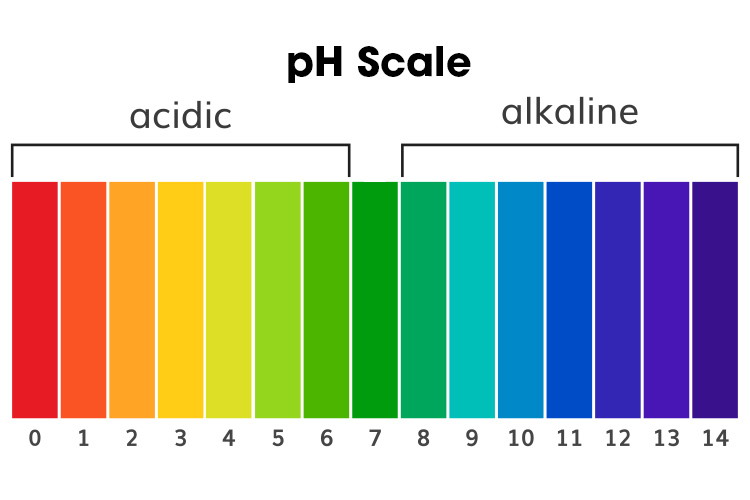
Alkalosis is the opposite. The higher the pH, the more base is in the blood sample, which can disrupt the normal functioning of the body.
Once you’ve determined whether there is too much acid or too much base, you can move on to determine the cause of it.
(Kaufman n.d.)
HCO3-: Respiratory or Metabolic?
After you’ve determined whether the sample is acidic or alkaline, you need to work out whether it’s due to respiratory or metabolic causes.
If the cause is respiratory in nature, the PaCO2 will be out of the normal range, whereas for metabolic problems, the HCO3- will be abnormal. Low PaCO2 points to respiratory alkalosis, and high HCO3- can indicate metabolic alkalosis.
(Kaufman n.d.)
PaCO2: Compensated or Uncompensated?
Compensation can be thought of as the body’s attempt at correcting an imbalance. Is one system in the body trying to compensate for an abnormality in another system? We can investigate this by looking at the opposing component of the problem.
For example, in an acidosis, we’d look at the level of HCO3-. Whereas, in an alkalosis, to determine whether the body is compensating, we’d look at what the PaCO2 is doing.
If the other level (or component) is within normal ranges, then the problem is non-compensated or uncompensated. Ultimately, the body is yet to fix the problem or has been unable to fix the problem.
However, if the other component has gone outside its normal reference ranges, we can think of it as compensation occurring (the body is trying to fix the problem). To assess how well it has been able to do this, we need to refer back to the pH. If the pH is not within or close to the normal ranges, then a partial compensation exists. If the pH is back within normal ranges, then full compensation has occurred.
A non-compensated or uncompensated abnormality usually represents an acute change occurring in the body.
And note - The terms partial and fully compensated are used to describe the level of compensation and do not necessarily mean the patient’s ABGs are normal or that they are healthy.
To Simplify...
| Compensated or Uncompensated? |
Respiratory or Metabolic? |
Acidic or Alkalotic? |
pH | PaCO2 | HCO3- |
|---|---|---|---|---|---|
| Respiratory | Acidosis | Low | High | ||
| Respiratory | Alkalosis | High | Low | ||
| Metabolic | Acidosis | Low | Low | ||
| Metabolic | Alkalosis | High | High | ||
| Compensated | Respiratory | Acidosis | Normal | High | |
| Compensated | Respiratory | Alkalosis | Normal | Low | |
| Compensated | Metabolic | Acidosis | Normal | Low | |
| Compensated | Metabolic | Alkalosis | Normal | High |
Case Study 1
Consider the following:
pH = 7.50
PaCO2 = 47
HCO3- = 32
Q1) Is it an acidosis or an alkalosis?
The pH is 7.50. This is higher than normal, so we have an alkalosis.
Q2) Is the problem of a respiratory or metabolic nature?
Where else is there an alkalosis? The HCO3- is 32, which is high. So, we have metabolic alkalosis.
Q3) Is there any compensation occurring? Has the body tried to fix the problem?
We need to look at the other component; in this case, what is the CO2? The CO2 is outside its normal ranges. It’s 47, which is high. So, the body is trying to fix the problem. However, the pH is not yet back within normal ranges, so a partial compensation exists.
Conclusion:
This ABG is an example of a partially compensated metabolic alkalosis.
Case Study 2
Consider the following:
pH = 7.30
PaCO2 = 50
HCO3- = 30
Q1) Is it an acidosis or an alkalosis?
The pH is 7.30. This is lower than normal, so we have an acidosis.
Q2) Is the problem of a respiratory or metabolic nature?
What else is acidotic? The CO2 is 50, which is high. So, we have respiratory acidosis.
Q3) Is there any compensation occurring? Has the body tried to fix the problem?
We need to look at the other component - HCO3- in this case. Is the HCO3- outside its normal ranges? Yes, normal HCO3- is between 22 and 26. So, the body is trying to fix this problem. Has the body done a good job of fixing the problem? Is the pH back within normal ranges? No, the pH is not within normal ranges, so there is partial compensation occurring.
Conclusion:
This ABG is an example of a partially compensated respiratory acidosis.
Note: ABGs should be thought of as a snapshot of how the body is interacting with its environment at a particular time. They should always be interpreted as part of a wider assessment of a patient’s respiratory function and in line with your organisation’s policies.
Topics
Further your knowledge
References
- Castro, D, Patil, SM, Zubair, M & Keenaghan, M 2024, 'Arterial Blood Gas', StatPearls, viewed 24 April 2025, https://www.ncbi.nlm.nih.gov/books/NBK536919/
- Chang, E, Daly, J & Elliot, D 2006, Pathophysiology Applied to Nursing Practice, 2nd edn, Mosby Australia, Port Melbourne, VIC, Australia.
- Gaines, K 2024, Arterial Blood Gases (ABGs) Explained, Nurse.org, 23 August, viewed 24 April 2025, https://nurse.org/articles/arterial-blood-gas-test/
- Kaufman, DA n.d., Interpretation of ABGs, American Thoracic Society, viewed 24 April 2025, https://www.thoracic.org/professionals/clinical-resources/critical-care/clinical-education/abgs.php
- Lippincott 2012, Pathophysiology Made Incredibly Easy!, 5th edn, Lippincott Williams & Wilkins, Ambler, PA, USA.
- MedlinePlus 2024, Blood Gases, U.S. Department of Health and Human Services, viewed 24 April 2025, https://medlineplus.gov/ency/article/003855.htm
- Nall, R 2023, 'Blood Gas Test', Healthline, 17 July, viewed 24 April 2025, https://www.healthline.com/health/blood-gases